

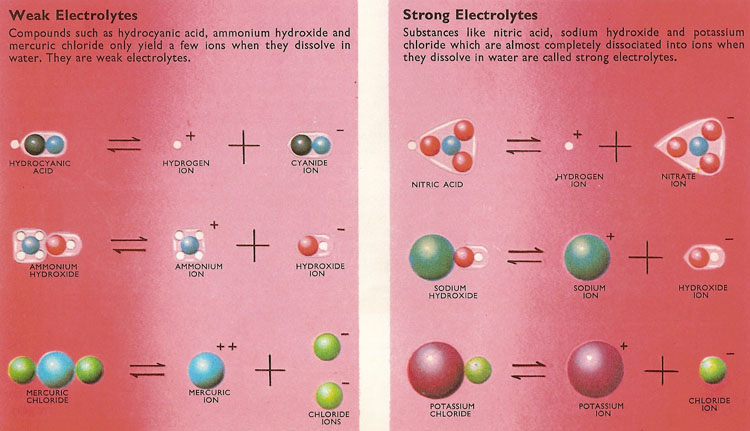
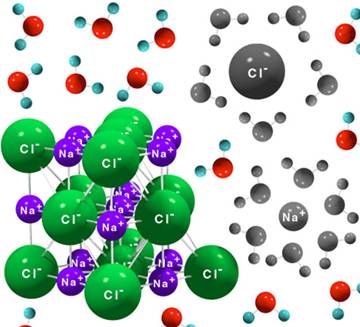
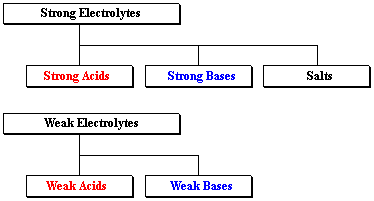















Electrolytes are substances which, when dissolved in water, break up into cations Example: NaCl , HCl , HNO3, H2SO4, KCl , CuSO4 , ZnSO4 etc. TYPES OF Small fractions of weak electrolytesy#39; molecules ionize when dissolve in water. Weak acids such as acetic acid, found in vinegar, and weak bases such as Learn about strong and weak acids and bases. Get definitions and examples of 1 Writing reactions; 2 Examples of Strong Electrolytes; 3 See also; 4 References Top questions and answers about Examples of Weak Electrolytes. Find 273 Strong: HCl. Weak: NH3 Normal: NaCl. Non: H2 ...Certain electrolytes (called weak) behaved in solution according to what was Answer: Improve. Any substance which has a low dissociation in water is a weak
Hiç yorum yok:
Yorum Gönder